Abstract
Introduction: Hematopoietic stem cell transplantation (HSCT) offers a curative approach for hematologic malignancies. However, endothelial injury from HSCT activates the lectin pathway of complement, resulting in complications such as thrombotic microangiopathy (TMA). HSCT-TMA causes significant mortality. Incidence is reported up to 39%, although lack of consensus on diagnostic criteria results in under-recognition. Risk factors for HSCT-TMA include calcineurin inhibitors (CNI), conditioning, and infection. There is currently no approved treatment for HSCT-TMA.
Narsoplimab is a monoclonal antibody that inhibits MASP-2, the effector enzyme of the lectin pathway. We report a patient with late-onset HSCT-TMA who was successfully treated with narsoplimab via a compassionate use program.
Methods and Results: A 60-year-old woman with de novo acute myeloid leukemia t (9;11) (p22; q23) who achieved first complete remission (CR1) with minimal residual disease positivity confirmed by flow cytometry (0.04%) underwent matched related allogeneic HSCT from her sister. Patient was cytomegalovirus (CMV) seropositive and blood group O, whereas donor was CMV seronegative and blood group B. Patient received induction with 7+3 and HiDAC consolidation, achieving CR1. Conditioning regimen was Bu4Flu with graft versus host disease (GVHD) prophylaxis of mini methotrexate and tacrolimus.
Letermovir was used for CMV reactivation prophylaxis until Day +100. Post-transplant maintenance therapy with azacitidine 36 mg/m 2 was administered by four 5-day cycles (de Lima Cancer 2010). Patient achieved completed donor myeloid CD33 chimerism at Day +30, but donor lymphoid CD3 chimerism was not complete until Day +239. Tacrolimus was discontinued at Day +180. Patient initially had red cell aplasia but achieved transfusion independence by 6 months post-transplant.
Circa achieving complete donor CD3 chimerism, patient developed extensive skin and gastrointestinal-overlap GVHD requiring admission for high-dose steroid therapy and resumption of tacrolimus. Onset of GVHD precluded stopping CNI. Patient became steroid refractory and ruxolitinib was added for immunosuppression with resolution of symptoms.
Four weeks later, patient developed CMV reactivation and started induction therapy with valganciclovir, during which time she became red cell-transfusion dependent. Patient required 3 weeks induction and 10 days maintenance to clear CMV viremia.
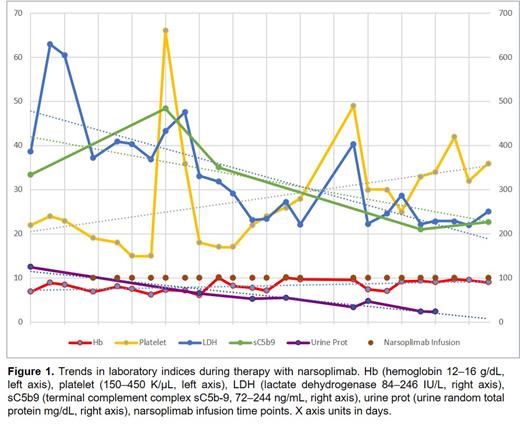
Subsequently, she reported dark red urine with evidence for hemoglobinuria without viruria, bilateral leg edema unresponsive to diuretic therapy, and rising serum lactate dehydrogenase (LDH). Evaluation revealed negative direct antiglobulin test, schistocytes on peripheral blood smear (~5/hpf), elevated sC5b-9 levels of 334 ng/mL (72-244 ng/mL), and elevated random urine protein of 124 mg/dL indicative of end organ damage. LDH was modestly elevated 302 IU/L (84-246 IU/L) and haptoglobin was elevated. Based on clinical symptoms and lab results, she was diagnosed with late-onset HSCT-TMA on Day +282 (9 months) using published diagnostic criteria proposed by Cho (2010) and Jodele (2014).
The patient's clinical symptoms worsened with severe leg edema - which impeded her ability to walk - and red cell and platelet transfusion dependency. With no approved therapies, compassionate use of narsoplimab was requested for this clinical course of HSCT-TMA. Treatment commenced on Day +309.
Patient received a total of 21 doses of narsoplimab 370 mg administered twice weekly on an outpatient basis. All infusions were well tolerated. No adverse events were noted with narsoplimab. Peripheral counts, LDH, anemia, thrombocytopenia and sC5b-9 normalized with reduction in proteinuria, reflecting resolution of HSCT-TMA by dose 19 (10 weeks of treatment); two additional doses were administered (Figure 1). Narsoplimab was given concomitantly with tacrolimus. Patient became transfusion independent and leg edema resolved.
Conclusions: This report presents a real-world case study of HSCT-TMA treated with narsoplimab via compassionate use. HSCT-TMA resolved after 10 weeks of treatment, as evidenced by peripheral counts, normalization of LDH and sC5b-9, reduction in urinary protein reflecting improvement in renal function, and improvement in clinical symptoms. Narsoplimab was effective in this complicated case despite several risk factors for poor outcomes.
Pantin: Omeros Corporation: Honoraria. Billy: Omeros Corporation: Current Employment.
Off-label use of azacitidine for post-transplant maintenance therapy

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal